Abstract
Epstein Barr virus (EBV)-associated lymphoproliferative disorder (LPD) is a major cause of morbidity and mortality in immunodeficient patients. This disorder has been extensively described after hematopoietic stem cell (SCT) or solid organ transplant. Since the 1980's, there have been isolated case reports of pediatric patients with acute lymphoblastic leukemia (ALL), who developed EBV-LPD during ALL therapy, without undergoing SCT. Comprehensive information is unavailable regarding the prevalence, clinical manifestations, treatment, outcome and pathogenesis of such disorders in this setting.
In this retrospective survey, national coordinators of International BFM (I-BFM) ALL study groups were asked to report patients ≤ 18 years of age who developed EBV-LPD during childhood ALL therapy since 2000. Patients with LPD following SCT were excluded. Complete information was requested regarding patient characteristics, leukemia and LPD characteristics, treatment and outcome. In addition, a literature search was performed, and patients described in previously published case reports were added to the series.
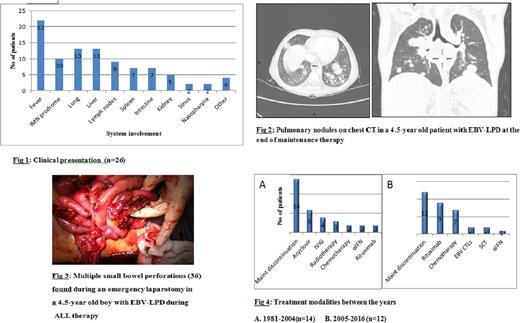
Ten cases of EBV-LPD during childhood ALL therapy were reported by I-BFM national coordinators. Another 16 case reports were identified by a literature search, thus forming a series of 26 patients. None of the patients reported a previous history of immune deficiency or cancer in the family. Eighteen of 26 patients were male. Median age was 4.5 years (range: 2 months-16 years). Eighteen patients were diagnosed with B-ALL, 3 with T-ALL (data unavailable for 5 cases). Only 2 cases received ALL treatment according to the high-risk (HR) arm of their respective protocols. All cases were diagnosed during maintenance therapy, or 1-2 months after its cessation. The median duration of maintenance exposure until LPD diagnosis was 12.5 months (range: 3-30 months). Patients presented with a diverse and often aggressive clinical picture, with frequent extra-nodal involvement (fig. 1). Of 26 patients, 22 patients presented with fever, 12 had an infectious mononucleosis (IMN)-like prodrome days to weeks before LPD diagnosis, 13 presented with pulmonary nodules (fig. 2) and 6 had multiple intestinal perforations (fig. 3). Pathology showed a spectrum of heterogenous lesions, as defined by the World Health Organization 2008 criteria for post-transplant LPD (PTLD), including only one case with an LPD of T-cell origin. In all cases maintenance treatment was discontinued. Other treatment modalities included chemotherapy, rituximab, EBV cytotoxic T-cell lymphocytes (CTLs) and SCT (fig. 4). In 5 patients maintenance was resumed, without LPD recurrence. Three patients died of EBV-LPD, 4 had an ALL relapse, 19 experienced continued remission from both ALL and LPD.
In this survey we describe the clinical manifestations, treatment and outcome of 26 patients who developed EBV-LPD during childhood ALL therapy. This is a very rare life-threatening disorder, but its exact incidence is difficult to estimate due to under-reporting, even among patients treated on clinical trials. The clinical presentation, range of pathological findings and treatment modalities were similar to PTLD. Most patients were diagnosed at a progressive stage of their disorder. Remarkably, all 26 cases were diagnosed during the maintenance phase of ALL treatment, after a median duration of 12.5 months. The majority were treated according to non-HR arms of therapy, where exposure to maintenance is usually of longer duration. There is limited information regarding immune function during maintenance therapy. EBV-LPD is a disorder resulting from proliferation of infected B-cells in the setting of deficient cytotoxic T-cell surveillance. Our findings suggest that the pathogenesis of this disorder is related to an immune dysregulation which occurs specifically under exposure to maintenance therapy, and that the B-cell and T-cell compartments may be differentially affected during this phase of therapy. These rare cases may enhance our understanding of the biology of EBV infection in immunodeficient patients. The vast majority of children with ALL do not develop EBV-LPD during maintenance therapy, suggesting a role for host genetic predisposition, which may be further investigated in future.
Schrappe: JAZZ Pharma: Consultancy, Research Funding; Baxalta: Consultancy, Research Funding; Medac: Consultancy, Research Funding; SigmaTau: Consultancy, Research Funding; Novartis: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal